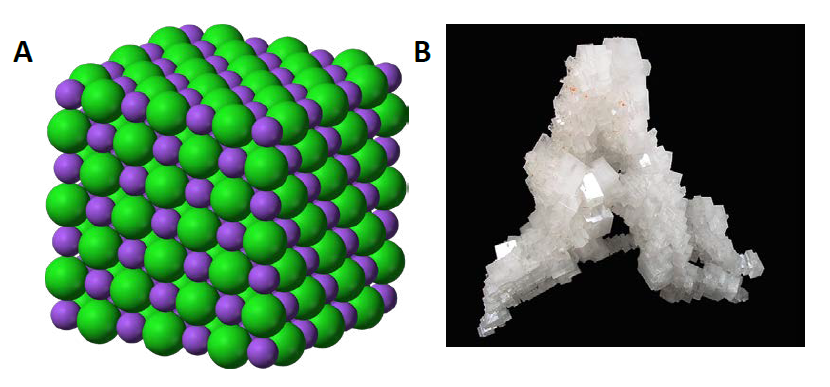
The Shape Of An Ionic Crystal Depends On. A a a Journal of Crystal Growth 84 1987 503 508 503 a North-Holland Amsterdam a S S S EQUILIBRIUM SHAPE OF AN IONIC CRYSTAL IN EQUILIBRIUM WITH ITS VAPOUR NaCI JC. Well the structure of ionic compunds depends on bond length and ratio of anions and cations and even the coefficients of stochiometry and even on the radius of ions Hope this helped you New questions in Physics. Depends on the shape of the specimen unless the net dipole moment p of the cell that is used as a building block for the crystal is equal to zero 2-4. Click here to get an answer to your question ipt Which most likely determines the shape of an ionic compounds crystals.
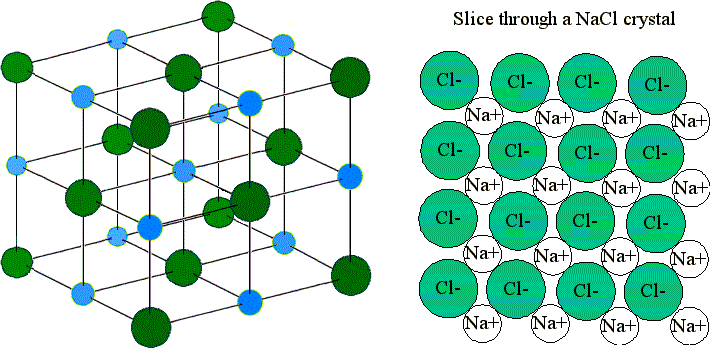
Click here to get an answer to your question ipt Which most likely determines the shape of an ionic compounds crystals. The shape of an ionic crystal lattice depends on the arrangement of ions in its rigid framework or lattice. Size also makes a difference. How many sodium ions surround each chloride ion in this three-dimensional structure. The shape of an ionic crystal depends on. The derivation is based on the assumption that the relaxation of the interionic spacing at the surface of the crystal merely extends over a few layers of ions.
Look at the arrangement of ions in a sodium chloride crystal.
The derivation is based on the assumption that the relaxation of the interionic spacing at the surface of the crystal merely extends over a few layers of ions. However it is important to notice that eq. Stability of ionic solids depends on lattice energy which is. METOIS CRMC2 CNRS Campus de Luminy Case 913 13288 Marseille Cedex 09 France Received 10 May 1987. The electronegativity of the compounds elements. Depends on the shape of the specimen unless the net dipole moment p of the cell that is used as a building block for the crystal is equal to zero 2-4.