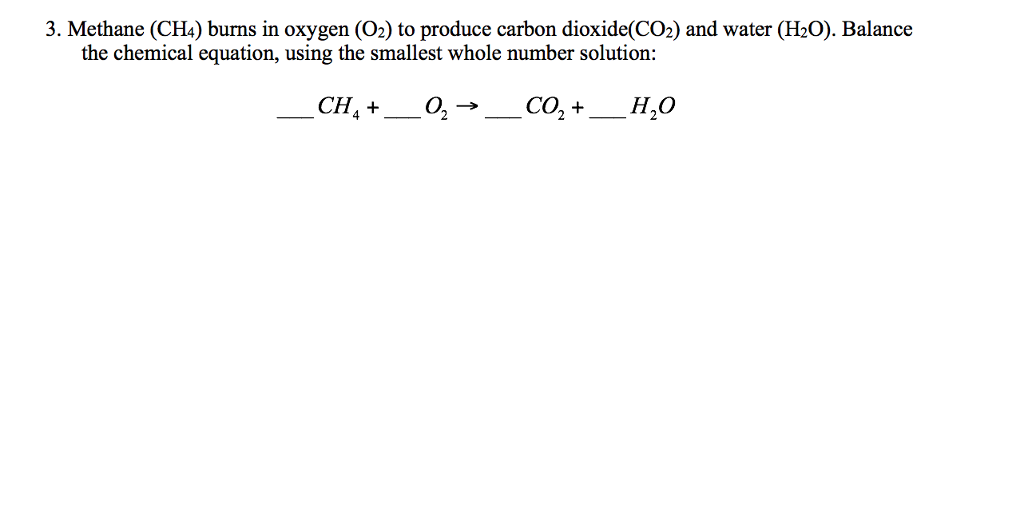
Methane Ch4 Burns In Oxygen. The balanced equation is. CH4 g 2 O2 g CO2 g 2 H2O g The balanced equation for the incomplete combustion of methane is. The unbalanced equation is. First be sure to count all of the O atoms on the products right side.
Multiple choice When methane is burned with oxygen the products are carbon dioxide and water. ˈ m iː θ eɪ n is a chemical compound with the chemical formula CH 4 one atom of carbon and four atoms of hydrogenIt is a group-14 hydride and the simplest alkane and is the main constituent of natural gasThe relative abundance of methane on Earth makes it an economically attractive fuel although capturing and storing it poses technical. Methane burns in oxygen to produce CO2 and H20. If you produce 18 grams of water from 8 grams of. According the coefficients in this equation two moles of ethane reacts with seven moles of oxygen. Response times vary by subject and question complexity.
According the coefficients in this equation two moles of ethane reacts with seven moles of oxygen.
ˈ m iː θ eɪ n is a chemical compound with the chemical formula CH 4 one atom of carbon and four atoms of hydrogenIt is a group-14 hydride and the simplest alkane and is the main constituent of natural gasThe relative abundance of methane on Earth makes it an economically attractive fuel although capturing and storing it poses technical. CH4 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. Methane CH4 can react with oxygen O2 to produce carbon dioxide CO2 and water H2O. Given a chemical reaction methane CH4 burns in oxygen O2 to produce carbon dioxide CO2 and water H2O. Methane burns in oxygen to produce carbon dioxide and water. The little number written at the lower right after an atom subscript tells how many of that atom are in the molecule.
