
Melting Point Dsc Curve. For breaking down the crystalline lattice. Equally important are the onset temperature Ton especially with the melting of pure materials and the. All samples showed an endothermic peak due to PP melting around 160. Some of these quantities are shown in the curve in Figure 1.
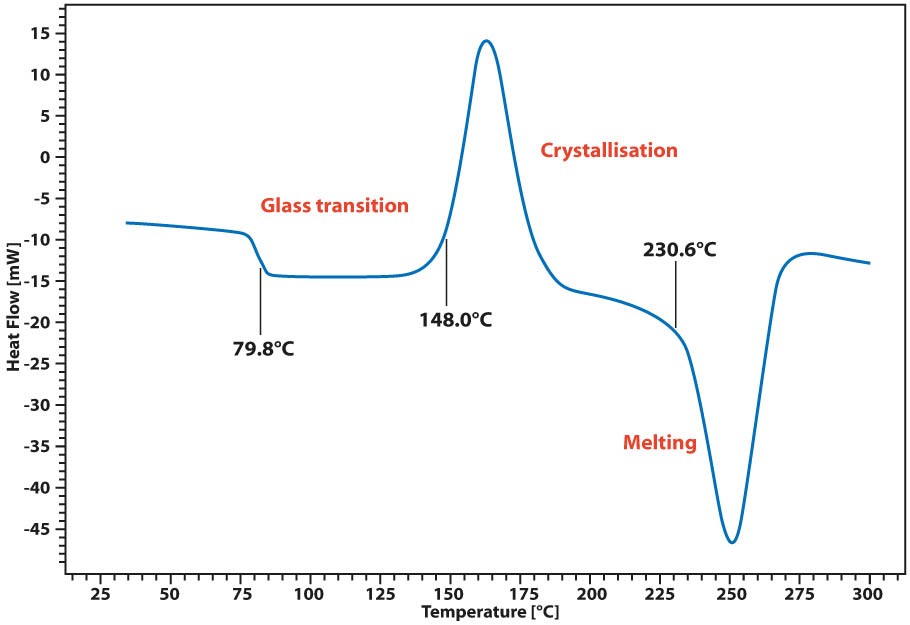
When you measure the melting point Tm in a DSC you get not only the onset of melting the Tm but also the peak temperature which corresponds to complete melting in organics and the energy that the melting transition needs in order to occur. This is the enthalpy of the transitions and it is associated with the crystallinity of materials. In DSC micrograph which points either onset point of melting or melting peak would indicate melting point. Di erential scanning calorimetry DSC is a technique used to investigate the response of polymers to heating. Volatilization can be detrimental to obtaining accurate quantitative results since sample mass changes. Theoretical DSC melting curves for small samples with sharp transition temperatures were created and compared with DSC melting curves from pure indium samples.
A sharp well defined melting.
Furthermore on the low temperature side of the endothermic peak the untreated sample showed a smooth DSC curve between 110 and 125 while the treated samples showed a minute peak. Interpreting DSC Data 8 Melting Point Peaks Melting peak characteristics will vary in response to many variables. DSC heating curve of 192 mg polystyrene showing a typical artifact at about 78 C caused by the thermal expansion of the Al pan. Some of these quantities are shown in the curve in Figure 1. The following characteristics were estimated from DSC curves. Temperature possibly along with one or more points where the sample is allowed to soak at a fixed temperature.