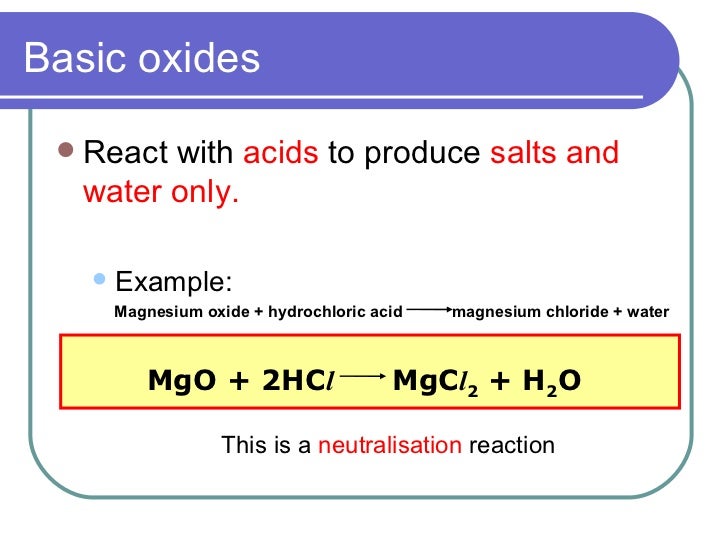
Magnesium Oxide And Phosphoric Acid. Magnesium hydroxide is soluble in dilute acid and ammonium salt solution. The liquid is essentially an aqueous solution of phosphoric acid buffered by adding small quantities of zinc oxide or aluminium oxide. Phosphoric acid reacts with magnesium hydroxide to produce magnesium phosphate and water via the following reactionH3PO4 MgOH2-Mg3PO42 H2O. Magnesium react with phosphoric acid 3Mg 2H 3 PO 4 Mg 3 PO 4 2 3H 2 Check the balance Magnesium react with phosphoric acid to.
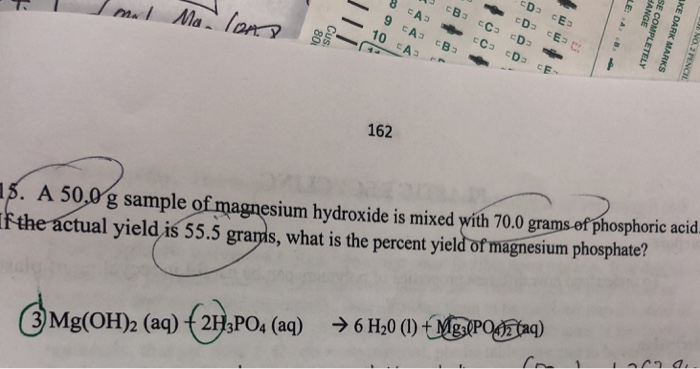
Solubility 18 is 00009 g. Phosphoric acid reacts with magnesium hydroxide to produce magnesium phosphate and water via the following reactionH3PO4 MgOH2 —– Mg3PO42 H2OBalance the. X H 2O x 0 to 4 Formula. Dimethyl Thio toluene Diamine. The aluminophosphate bond to a greater degree contributes to recrystallization of the periclase compared with H3PO4 magnesium- and chromium-phosphate bonds. 3Mgs 2H3PO4aq — 3H2g Mg3PO42sI believe the equation is.
Magnesium hydroxide react with phosphoric acid.
Chemical reactions Сhemical tables. For example C6H5C2H5 O2 C6H5OH CO2 H2O will not be balanced but XC2H5. In the sodium oxide the solid is held together by attractions between 1 and 2- ions. Magnesium hydroxide is soluble in dilute acid and ammonium salt solution. For example it reacts with warm dilute hydrochloric acid to give magnesium chloride solution. Magnesium Oxide Phosphoric Acid Magnesium Phosphate Water Bi2O3 ClO- OH- BiO3- Cl- H2O.