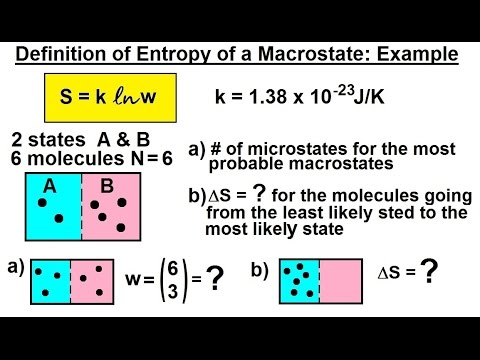
Macrostate And Microstate In Statistical Mechanics. There The entropy can be de ned as a thermodynamic observable with no reference to an un-derlying statistical model but it is much more instructive to derive it from microscopic considerations. A macrostate tells us nothing about a state of an individual particle. Descrition of macrostate implies specifying fewer variables such as energy temperature. N r.

There The entropy can be de ned as a thermodynamic observable with no reference to an un-derlying statistical model but it is much more instructive to derive it from microscopic considerations. For a classical particle 6 parameters x. Descrition of macrostate implies specifying fewer variables such as energy temperature. Classical statistics or Maxwell-Boltzmann statistics. Since for the macrostate r n r the number of microstates is n r n. In contrast the macrostate of a system refers to its macroscopic properties such.
In contrast the macrostate of a system refers to its macroscopic properties such.
A statistical experiment can result in an outcome. Created by Sal Khan. The key difference between microstate and macrostate is that microstate refers to the microscopic configuration of a thermodynamic system whereas macrostate refers to the macroscopic properties of a thermodynamic system. The state of a system specified by describing the quantum state of each molecule in the system. A macrostate is defined by the macroscopic properties of the system such as temperature pressure volume etc. Microstates Consider an isolated system for example our typical ther-modynamic system shown in Fig.