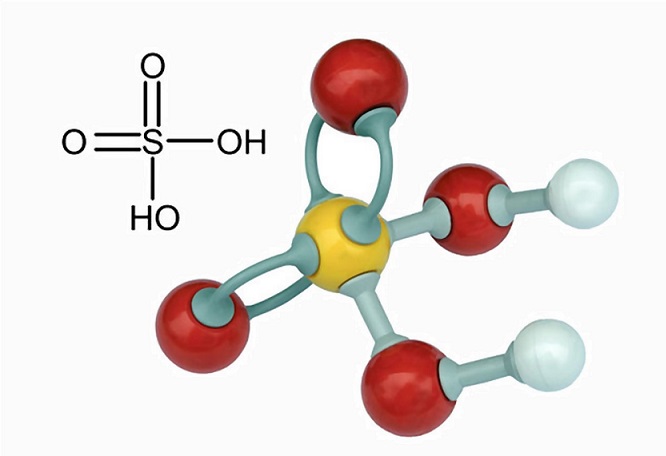
Is Sulfuric Acid Polar. Ill tell you the polar or nonpolar list below. Find this compound at Sigma-Aldrich to meet your research needs. Sulfuric acid H2SO4 or H2O4S CID 1118 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. H2SO4 SULFURIC ACID is Polar.

Is sulfuric acid an ionic or covalent bond. The sulfuric acid formula H 2 SO 4 indicates the presence of a sulfur atom surrounded by two hydroxide compounds and two oxygen atoms. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar. Find this compound at Sigma-Aldrich to meet your research needs. Answer H2SO4 SULFURIC ACID is Polar What is polar and non-polar. A polar molecule with two or more polar bonds must have an asymmetric.
If you will see the end of the acid you will see a carboxylic acid group which is highly polar that is it will be dissolved in water.
This is because of the HYDROPHOBIC CARBON TAIL that they possess which repels water. Ill tell you the polar or nonpolar list below. Sulfuric acid H2SO4 is a covalent bond What is chemical bond ionic bond covalent bond. This powerful acid is used in various industries. The sulfuric acid formula H 2 SO 4 indicates the presence of a sulfur atom surrounded by two hydroxide compounds and two oxygen atoms. Sulfuric acid H2SO4 is a Molecular bond What is chemical bond ionic bond Molecular bond.