Chlorobenzene To Phenol Mechanism. Benzene is first of all converted into chlorobenzene by treating it with chlorine in presence of anhydrous AlCl₃ or FeCl₃. In addition aryl chlorides bromides and iodides can be converted to areneamines ArNH 2 by the conjugate bases of amines. NaOH OH replaces the Chlorine in the benzene ring and thus Phenol is. When thus obtained chlorobenzene is treated with aq.

This process is known as Dows process. And in second step chlorobenzene is to be treated with NaOH at about 623 K and 300 atm pressure to form sodium phenate sodium phenoxidewhich is acidified by dilute HCl to form phenol Daws process 22K views. In the Dow process chlorobenzene is reacted with dilute sodium hydroxide at 300C and 3000 psi pressure. Formation of phenol from chlorobenzene is an example of. Phenol Synthesis from Chlorobenzene - YouTube. By using Dows Process conversion of Chloro benzene to phenol is explained.
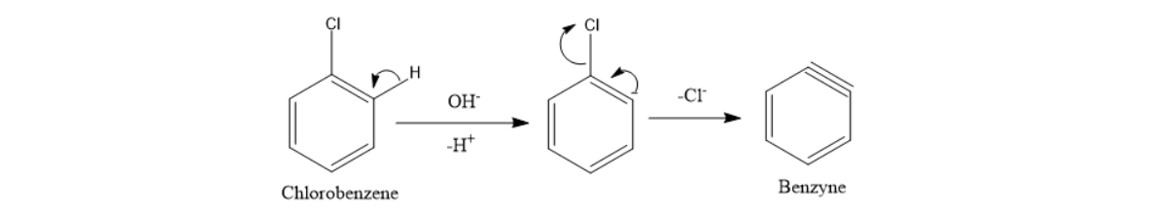
Chlorobenzene forms benzyne by removal of H by the attack of base then removal Cl -.
This process is known as Dows process. NaOH OH replaces the Chlorine in the benzene ring and thus Phenol is. OH on the benzene ring. This reacts with H-OH or HCl where H is taken by negatively charged ortho carbon and formation of phenol takes place. Chlorobenzene forms benzyne by removal of H by the attack of base then removal Cl -. Following is a two-part question based on the nucleophilic aromatic substitution reaction below which converts chlorobenzene to phenol.