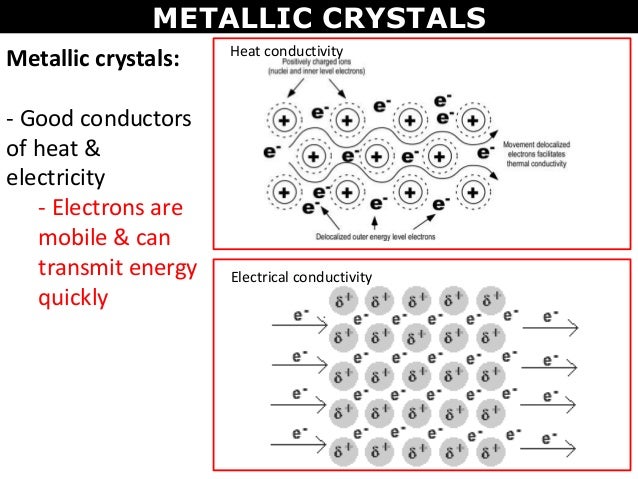
Characteristics Of Metallic Crystals. The proper-ties one from the force perspective the other from energy should be the same. Because outer electrons of metal atoms are delocalized and highly mobile metals have electrical and thermal. Iron Copper Alluminium Zinc Magnesium etc. They are highly malleable and ductile.

Electrostatic forces form ionic bonds. Figure 3 left panel. The proper-ties one from the force perspective the other from energy should be the same. Metal crystals describe the crystal structures that are found in metals. Iron Copper Alluminium Zinc Magnesium etc. Metallic hydrogen is a phase of hydrogen in which it behaves like an electrical conductor.
In general metals are denser than nonmetals.
This leads to electrical conduction. Because outer electrons of metal atoms are delocalized and highly mobile metals have electrical and thermal. The isolated metallic cubes are less lossy than the connected rod structures. Have the simplest crystal structures. Reflection properties of metallic photonic crystals Reflection properties of metallic photonic crystals Temelkuran B. They are highly malleable and ductile.