Atomic Structure Of Beryllium. Beryllium is widely used to alloy with other metals such as copper and is used in springs and electrical equipment due to its non magnetic nature. Diagram of the nuclear composition electron configuration chemical data and valence orbitals of an atom of beryllium-9 atomic number. Beryllium chemical element that is the lightest member of the alkaline-earth metals of Group 2 of the periodic table. The chemical symbol for Beryllium is Be.

Hexagonal Density 293 K. The total electrical charge of the nucleus is therefore Ze where e. It is used in metallurgy as a hardening agent and in many outer space and nuclear applications. Diagram showing the nuclear composition and electron configuration of an atom of beryllium-9 atomic number. Beryllium chemical element that is the lightest member of the alkaline-earth metals of Group 2 of the periodic table. Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structure.
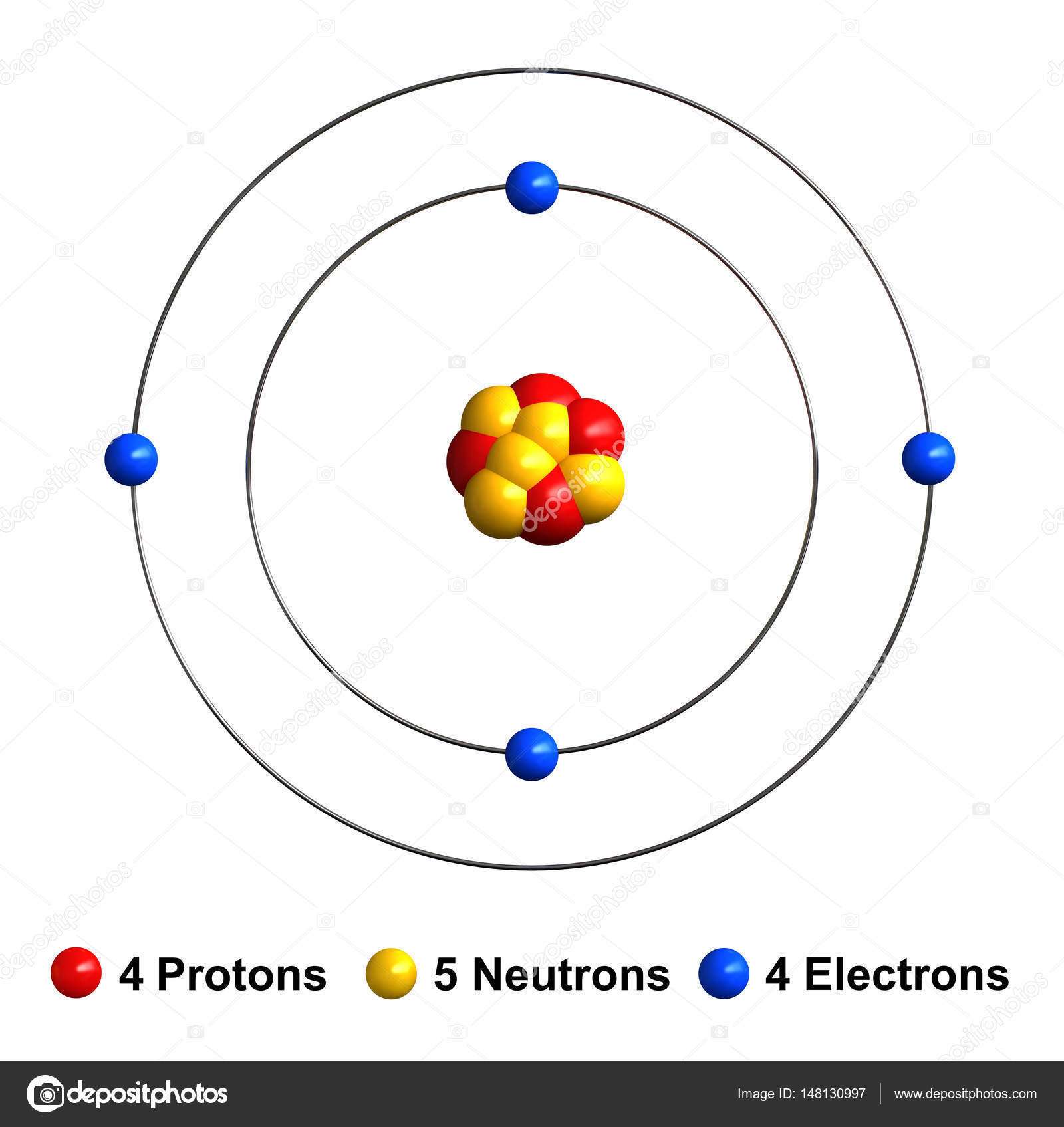
Four electrons green bind to the nucleus successively occupying available electron shells rings.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Beryllium Be is a light silver-gray metal that has the atomic number 4 in the periodic table. Diagram of the nuclear composition electron configuration chemical data and valence orbitals of an atom of beryllium-9 atomic number. The nucleus consists of 4 protons red and 5 neutrons orange. Alkaline Earth Crystal Structure. 4 Number of Neutrons.