
An Iron Ore Contains 38 Fe2o3 By Mass. 5 points An iron ore contains 38 Fe203 by mass What is the maximum mass in kg of iron that can be recovered from 100 kg of. An iron ore sample contains Fe 2 O 3 plus other impurities. What is the maximum mass of iron that can be recovered from 100 kg of this ore. An iron ore contains 38 Fe203 by mass.
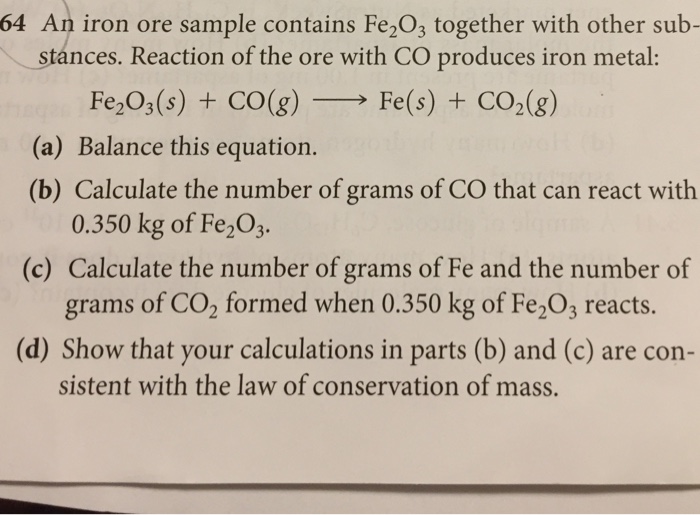
Get the detailed answer. The iron in a 389-gram sample of the ore is all converted by a series of chemical reactions to. The mass of impure Fe2O3 or the mass of the iron ore sample is already given as 752 g. Fe 2 O 3 s 3 C s 2 Fe s 3 CO g What is the mass percent of Fe 2 O 3 in the impure iron ore sample. Here we are going to calculate the maximum mass of iron that can be recovered from 100 kg of the ore step 1 given 38 of fe2o3 by mass mass of fe2o3 in 10kg x 10 x 1000 g 3800 g 38 kg of fe in fe2o3 0699. 5 points An iron ore contains 38 Fe203 by mass What is the maximum mass in kg of iron that can be recovered from 100 kg of.
An ore contains Fe3O4 and no other iron.
An ore contains Fe3O4 and no other iron. A particular sample of iron ore contains 85 by mass Fe2O3 Mr1596 and no other iron compound. What is the maximum mass of iron that can be recovered from 100 kg of this ore. Here we are going to calculate the maximum mass of iron that can be recovered from 100 kg of the ore step 1 given 38 of fe2o3 by mass mass of fe2o3 in 10kg x 10 x 1000 g 3800 g 38 kg of fe in fe2o3 0699. A 752-g sample of impure iron ore is heated with excess carbon producing 453 g of pure iron by the following reaction. Fe2O3 s 3COg 2Fel 3CO2g.